what is the nature of new elements added to the periodic table in the last hundred years?
The Newest Elements on the Periodic Tabular array
The symbols and atomic numbers for the chemical elements Nihonium, Moscovium, Ognassen and Tennessine (Dr_Micbrobe, iStockphoto)
How does this align with my curriculum?
If you discovered a new element, what would you name it? Would you name it after your favourite Boob tube character? Your hometown? Yourself? The International Matrimony of Pure and Applied Chemistry (IUPAC) sets guidelines on naming chemic elements. They say that elements can be named subsequently mythical characters, concepts, minerals, places, element backdrop, or scientists. Nonetheless, in one case a proper name and symbol have been chosen, they can never exist changed. So decide carefully!
In 2016, iv new elements were added to the periodic table of elements. Let's acquire a piffling bit nigh these newest elements, and what they were named. But first, let'due south learn about what chemic elements are.
What are chemical elements?
Chemical elements are the edifice blocks of chemical science. They brand up all of the ordinary matter of the universe. For example, oxygen is an element. It is the third-almost arable element in the universe. You can find information technology in water (H2O) and many other molecules that living organisms rely on.
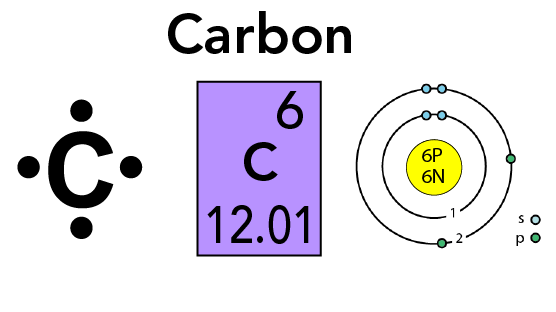
Each element has its ain diminutive number. The atomic number tells you the number of protons in an element. For instance, carbon has an atomic number of half-dozen. This means it has vi protons. Protons take a positive electric charge and are contained in the nucleus. The nucleus is a dense region located in the center of an atom.
Elements can also contain neutrons and electrons. Neutrons are a type of particle with approximately the aforementioned mass as a proton. Unlike protons, neutrons practice not have any electrical charge. Just similar protons, they are plant in the nucleus.
Electrons have a negative electrical charge. They are also much smaller and lighter. They orbit effectually the nucleus.
Did you lot know?
Atoms of a certain chemical element have the same number of protons but tin can accept dissimilar numbers of neutrons. These variants are chosen isotopes.
What is the periodic table?
The periodic table of elements is a tabular array that arranges the chemical elements in a logical way. They are arranged based on their atomic number, electron configurations, and chemical properties. The rows of the periodic table are chosen periods. The columns are called groups.
The periodic table that nosotros use today contains vii periods and 18 groups. This design lets you lot apace detect an element's symbol, atomic number, and atomic mass.
The table tin as well give you data on chemical properties. For example, elements on the left-paw side of the periodic tabular array are by and large metals. The elements on the right-hand side are generally not-metals.
When was the periodic tabular array of elements last updated?
In 2016, four new elements were added to the periodic tabular array. That means the seventh flow of the periodic table is complete.
Those iv elements are numbered 113, 115, 117 and 118. The enquiry groups that discovered them were from Nihon, Russia, and the United States. Call up how we said elements could be named after a place? Well, three of these elements were named afterwards the places where they were discovered. Their names are Nihonium, Moscovium and Tennessine. The fourth element is named Oganesson. Information technology was named later on a Russian nuclear physicist named Yuri Oganessian.
What are super heavy elements?
All four of the newest elements are highly unstable super heavy metals. Heavy elements are those that take an diminutive number larger than 92. Super heavy elements usually have atomic numbers larger than 112. Super heavy elements are besides more radioactive and unstable than other elements.
Super heavy elements do not occur in nature. The new elements were created in laboratories. Scientists apply machines called particle accelerators to make ions (a charged element) of one element crash into the ions of another chemical element. Ions are charged atoms. When the nuclei crash together, they may join together. If the nuclei join, a new element is created. Simply these artificially created elements only exist for a fraction of a second earlier they decay into other elements.
Did you know?
Particle accelerators propel charged particles to speeds close to the speed of light.
Producing new elements is very difficult. The new elements all disuse speedily. This happens because their nuclei are packed with a large number of protons. Protons are positively charged, and then they repel each other. This makes the atoms highly unstable. For case, Nihonium has a one-half-life of but 10 seconds. As the elements disuse, they release particles and free energy.
Did you lot know?
Heavy metals aren't actually heavy at all! The term "heavy metal" refers to the element's high atomic weight (92-102) and high density.
Why were these four elements such a big deal when they were discovered?
The newest heavy elements are important both scientifically and practically. Scientifically, the discovery tin give scientists a better understanding of how nuclei are held together. This could, for case, lead to the evolution of safer and more efficient nuclear reactors.
Previously discovered heavy elements have also had practical applications. For example, Americium (Am) has been used in fume detectors. Plutonium (Pu) has been used in nuclear weapons and as well to power unmanned space probes.
Right now, the four newest chemical elements are used but in research. But scientists expect that they will discover applied applications for them in the near future.
Did y'all know?
The first artificial heavy element was created using particle accelerators at the Academy of California at Berkeley. This element, with atomic number 93, is at present known as neptunium.
Although the 7th menstruum of the periodic table of elements is now complete, the table itself may not be fully complete. Some scientists experience at that place are no limits to the periodic table. No ane is certain how long it will take, merely it is certainly possible for new elements to be discovered in the future. The periodic tabular array of elements could one day take a whole new 8th row. If this happens, it'll be time for a new periodic table poster in your chemical science classroom!
Connecting and Relating
- Which elements from the periodic table do you utilise in your everyday life?
- If you could name a new chemical element, what would you lot call information technology and why?
- What do yous think is the virtually interesting chemical element name? Why?
Connecting and Relating
- Which elements from the periodic table practise you apply in your everyday life?
- If you could proper name a new element, what would you telephone call it and why?
- What practise you think is the nearly interesting chemic element name? Why?
Relating Science and Technology to Club and the Environs
- Fifty-fifty though element 113 had been created twice before, it took seven years to create it again. Is creating new elements worth the time and financial investment? Explicate.
- How has the evolution of new technologies contributed to the discovery of new elements? Explain.
Relating Science and Technology to Gild and the Environment
- Even though chemical element 113 had been created twice earlier, it took seven years to create information technology once more. Is creating new elements worth the time and financial investment? Explain.
- How has the development of new technologies contributed to the discovery of new elements? Explicate.
Exploring Concepts
- Are there however more elements to be discovered? Explain.
- What is the process for naming new elements once they have been discovered?
- What are the benefits of studying super heavy metals? Why would scientists spend time creating them?
Exploring Concepts
- Are there still more elements to be discovered? Explain.
- What is the process for naming new elements one time they have been discovered?
- What are the benefits of studying super heavy metals? Why would scientists spend time creating them?
Nature of Science/Nature of Technology
- How does the periodic table of elements demonstrate that 'scientific knowledge is tentative and subject to modify'?
- Should scientists be awarded money when they discover a new element? Why or why not?
- The 4 elements mentioned in the article were discovered by different groups of scientists who were from Japan, Russia, and the United States. Does this fact marshal with how you normally remember of how scientists work? Explain.
Nature of Scientific discipline/Nature of Technology
- How does the periodic table of elements demonstrate that 'scientific knowledge is tentative and subject field to change'?
- Should scientists be awarded money when they discover a new element? Why or why non?
- The four elements mentioned in the article were discovered by dissimilar groups of scientists who were from Japan, Russia, and the Usa. Does this fact align with how you normally think of how scientists work? Explain.
Instruction Suggestions
- This article supports teaching and learning in Chemistry and Nature of Science. It focuses on the development of the periodic table, the discovery of chemical elements and basic atomic structure. Concepts introduced include atomic number, protons, neutrons, electrons, menstruum, heavy metals and super heavy metals.
- To introduce this topic, teachers could atomic number 82 students in a grouping word, to review the footing of the periodic table - what is it, how it was created, how it exists, why it is of import, etc.
-
Alternately, earlier having students read the commodity, teachers could take them consummate the 'Before' section of the Anticipation Guide learning strategy for this article. After reading and viewing, teachers could have students complete the 'Later on' section of the Anticipation Guide. Students could discuss their responses and clarify any questions that they may still have. Ready-to-use reproducibles for this strategy can be downloaded in [Google medico] and [PDF] formats. The Answer Key is bachelor as a [PDF].
- To follow up, the teacher could lead a group discussion on the discovery of new elements on the periodic tabular array and what this might mean for the future.
- To conclude, students could complete an Go out Sideslip to consolidate their understanding of the video and topics discussed. Teachers could collect and review the answers provided to assess the depth of student understanding on this topic. The ready-to-apply Go out Slip reproducible for this video can be downloaded in [Google doc] and [PDF] formats.
Educational activity Suggestions
- This commodity supports education and learning in Chemistry and Nature of Scientific discipline. It focuses on the development of the periodic table, the discovery of chemical elements and bones atomic structure. Concepts introduced include atomic number, protons, neutrons, electrons, period, heavy metals and super heavy metals.
- To innovate this topic, teachers could lead students in a group discussion, to review the basis of the periodic table - what is it, how information technology was created, how it exists, why it is important, etc.
-
Alternately, before having students read the article, teachers could have them complete the 'Before' department of the Anticipation Guide learning strategy for this article. After reading and viewing, teachers could take students consummate the 'After' section of the Anticipation Guide. Students could discuss their responses and clarify any questions that they may still have. Ready-to-use reproducibles for this strategy tin can be downloaded in [Google doc] and [PDF] formats. The Answer Key is available as a [PDF].
- To follow up, the teacher could lead a group give-and-take on the discovery of new elements on the periodic table and what this might hateful for the future.
- To conclude, students could complete an Get out Slip to consolidate their understanding of the video and topics discussed. Teachers could collect and review the answers provided to appraise the depth of pupil agreement on this topic. The fix-to-use Exit Sideslip reproducible for this video can be downloaded in [Google doc] and [PDF] formats.
Let'southward Talk Science needs your input!
Our programs and resource are offered at no price thanks to generous contributions from our supporters.
Please take a minute to tell united states the proper name of your school/arrangement so we can ameliorate understand the bear on of our programs.
Open Survey
Source: https://letstalkscience.ca/educational-resources/stem-in-context/newest-elements-on-periodic-table


0 Response to "what is the nature of new elements added to the periodic table in the last hundred years?"
Enviar um comentário